As part of its ongoing commitment to excellence Florence Cosmetics Manufacturing Ind. L.L.C is to ensure profitability, growth and continuous improvement and customer satisfaction through manufacturing and supplying quality products and services. Florence cosmetics is also committed to develop its people and the work environment through training and modern technologies in application of relevant standard and regulations.
The QMS Policy of Florence Cosmetics Manufacturing Ind. L.L.C remains committed to the “Manufacturing of Cosmetics Products” considering the following QMS policy has been established;
Quality objectives are strategic, apply to the entire Company and shall:
Quality Performance Objectives are measurable targets for improving operational performance to ensure process conformity and customer satisfaction. They apply to all departments and functions having direct responsibility for activities that require improvement. Performance objectives and goals are established by management and through employee involvement and monitored within the framework of management reviews.
Florence Cosmetics Manufacturing Ind. L.L.C retains documented information on the status of our quality objectives. If shortfalls are identified, management may revise objectives, issue corrective action requests, or take other appropriate actions to address the issue.
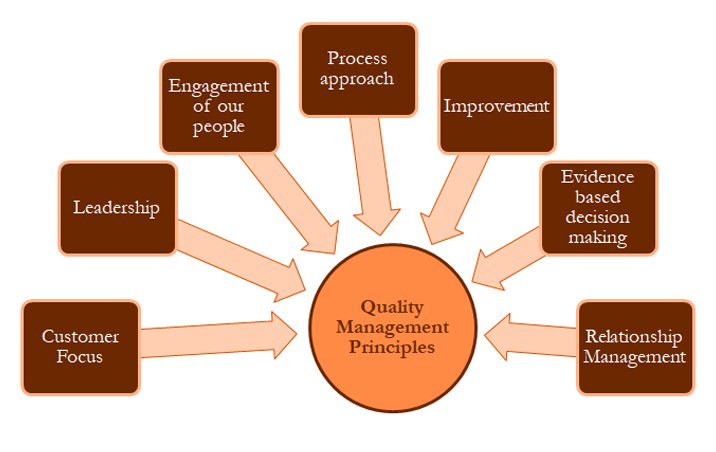
Florence Cosmetics Manufacturing Ind. L.L.C has adopted and realizes benefits of quality management principles into our daily activities. The intent of the quality management system is to provide a foundation to continually improve upon the company’s performance. Subsequent sections of the quality manual will provide our commitment of the following QMP elements:
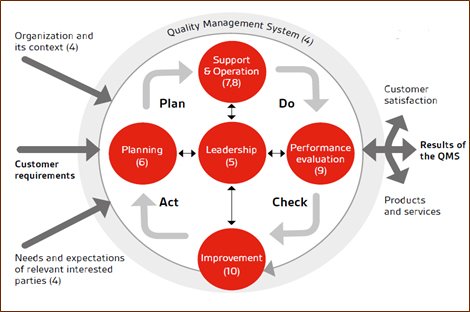
Florence Cosmetics Manufacturing Ind. L.L.C has established, implemented, controlled and maintained the processes needed to meet QMS requirements, and to implement the actions identified by:

Florence Cosmetics Manufacturing Ind. L.L.C has adopted the “Process Approach” into our daily operation including the PDCA Cycle. We have considered the utilization of RISK BASED THINIKING Philosophy when developing, implementing, and improving the effectiveness of our quality management system. This approach will enable Automation Solution to enhance the overall performance of the company by effectively controlling the interrelationships and interdependencies among the QMS processes. The implementation of the “Process Approach” in our QMS enables;